
Catalytic converters are a key component of modern automobiles and other vehicles, playing an essential role in reducing harmful emissions.
They serve as powerful filters that help to break down pollutants like carbon monoxide, hydrocarbons, nitrogen oxides, and other noxious gases into less toxic compounds.
The science behind catalytic converters is complex but fascinating; it involves the use of precious metals such as palladium, rhodium, or platinum which act as agents to create chemical reactions to purify exhaust fumes.
This technology has been instrumental in improving air quality worldwide and protecting human health from the potentially damaging effects of vehicle pollution. In this article, we will explore how catalytic converters work and why they are so effective at reducing emissions.
The Chemistry of Catalytic Converters

The Chemistry of Catalytic Converters is a crucial part of understanding how catalytic converters work to reduce emissions.
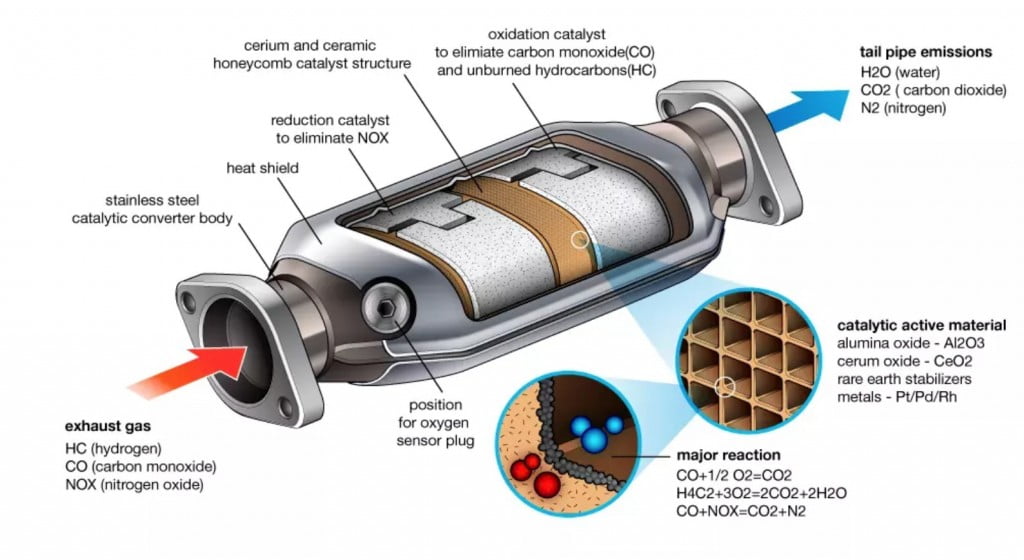
In general, catalytic converters are comprised of three main components: an oxidation catalyst, a reduction catalyst, and a substrate or support material.
The oxidation catalyst converts carbon monoxide and hydrocarbons into water vapor and carbon dioxide while the reduction catalyst reduces nitrogen oxides into harmless gases like nitrogen and oxygen.
The substrate helps to hold the other two components together allowing them to interact with one another more easily. To understand the chemistry behind this process it is important to look at each component’s roles within the catalytic converter system.
For example, when fuel enters the combustion chamber it contains large amounts of unburned hydrocarbon molecules that need to be broken down for efficient combustion – this job is done by the oxidation catalyst which works by breaking down these molecules into smaller ones that can then be burned more efficiently thus reducing emissions from vehicles significantly.
On the other hand, when exhaust gases exit they contain large amounts of harmful pollutants such as nitrogen oxide (NOx) which must be reduced before entering back into the atmosphere – this task is handled by a reduction catalyst which works by converting NOx molecules into simpler components like nitrogen gas and oxygen gas.
Finally, without a proper substrate, these two processes could not take place effectively so its role should not be overlooked either!
In conclusion, understanding the chemistry behind catalytic converters is essential for us to comprehend their effectiveness in reducing harmful emissions from vehicles today – ultimately providing us with cleaner air quality around our cities and towns!
How Catalytic Converters Reduce Harmful Emissions
Catalytic converters are devices designed to reduce the amount of harmful emissions released from internal combustion engines.
They work by breaking down pollutants, such as carbon monoxide and nitrogen oxides, into less toxic compounds before they are expelled from the vehicle’s exhaust pipe.
The catalysts used in these converters facilitate this process through a chemical reaction that requires very high temperatures to be effective.
Through this process, catalytic converters make it possible for cars and other vehicles to comply with environmental regulations while still providing efficient performance.
Catalytic converters have become an indispensable tool in reducing air pollution caused by motor vehicles and helping us build a healthier environment for all of us.
Benefits of Using Catalytic Converters

One of the main benefits of using catalytic converters is their ability to reduce harmful emissions from vehicles.
When a vehicle’s exhaust gases pass through the converter, chemical reactions occur which convert hazardous pollutants into less toxic substances. This helps to keep air quality in check and prevents smog formation.
Additionally, modern catalytic converters also help with reducing carbon dioxide emissions, making them an important tool for fighting climate change. Furthermore, these devices can improve a car’s fuel efficiency as well as its performance by decreasing the amount of energy lost during combustion.
All in all, catalytic converters are essential components that make it possible for vehicles to meet emission standards while still providing an enjoyable driving experience.
Conclusion

Catalytic converters are an essential piece of technology in reducing harmful emissions from vehicles. They work by using catalysts to break down pollutants in the exhaust into less hazardous compounds before they escape into the atmosphere.
This not only helps reduce air pollution but also makes cars run more efficiently and extends their lifespan.
As a bonus, installing a good quality cat-back exhaust system, such as those featuring high-quality stainless steel exhaust tips, can improve your car’s performance even further for a fraction of the cost!



