Paragard, a hormone-free intrauterine device (IUD), has long been promoted as a convenient, long-term birth control solution. Since its introduction in 1984, Paragard has been marketed as a safe and effective contraceptive.
Recently, the device has come under intense scrutiny. This is due to a wave of lawsuits filed by women who allege severe complications during its removal. A growing number of women claim that the device’s design flaw has led to painful and life-altering consequences upon removal.
According to Drugwatch, there are over 2,000 lawsuits pending against the manufacturer in the court of Georgia. To speed up the process, they have been consolidated in a multidistrict litigation.
In this article, we talk about the Paragard lawsuits, exploring the risks associated with this IUD. We will also discuss the ongoing multidistrict litigation (MDL) and the potential implications for medical device safety.
Paragard – A Non-Hormonal Birth Control
As noted by Mayo Clinic, Paragard stands as a unique contraceptive choice in the United States. It offers hormone-free birth control that lasts up to a decade. Many users appreciate its hormone-free approach to birth control.
Its mechanism involves copper, not hormones, to prevent pregnancy by interfering with sperm movement and egg implantation. Despite its long-standing presence in the market, concerns have arisen about its safety during removal.
Paragard Lawsuits Emerge
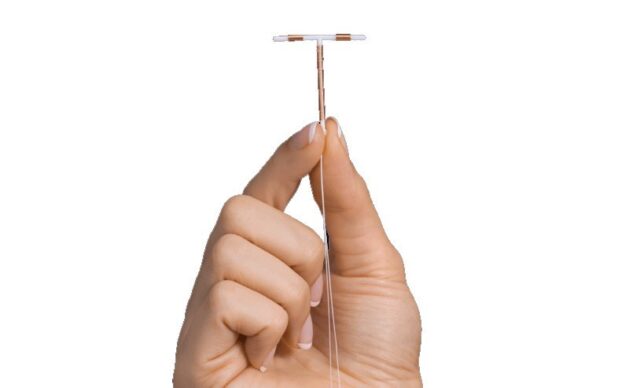
An increasing number of women have filed lawsuits against Paragard’s manufacturer, Cooper Surgical. The lawsuits allege that the IUD can break during removal, causing complications such as pain, perforation, and even infertility.
Many plaintiffs have required surgery to extract the broken pieces, prompting questions about the device’s safety. Women claim Cooper Surgical failed to notify them about the potential of Paragard breaking during removal. The Paragard lawsuit also claims that it is a defective medical device.
Allegations of Negligence and Defects
Plaintiffs argue that the companies should have been more forthright about the potential complications associated with Paragard, especially during removal. According to TorHoerman Law, plaintiffs claim that they were not adequately informed of the risks. This prevented them from making fully informed decisions about their birth control options.
The lawsuits mention that issues in the production process may have contributed to the IUD’s tendency to break during removal. This allegation underscores the importance of rigorous quality control in medical device manufacturing.
Risks and Injuries
The reported injuries associated with Paragard removal have had a profound impact on the lives of affected women. Infections resulting from IUD complications can lead to serious health issues, including pelvic inflammatory disease, which can cause infertility.
Additionally, the breakage and migration of IUD fragments into nearby organs can require invasive surgical procedures. This can lead to physical and emotional distress. Potential side effects, such as bacterial vaginosis and irregular menstrual bleeding, add another layer of complexity to Paragard’s safety profile.
Regulatory Oversight and Recalls

The FDA’s role in monitoring the safety of medical devices like Paragard is essential for protecting patients. The fact that thousands of device breakages were reported to the FDA underscores the need for ongoing surveillance.
According to The Legal Examiner, the Paragard IUD has already been recalled once in 2014 due to sterility issues. This previous recall serves as a stark reminder of the potential pitfalls in medical device manufacturing. Although no injuries were reported in this case, it highlights the critical need for quality control and rigorous testing.
Seeking Compensation and Future Developments
Women harmed by Paragard may seek compensation for physical, financial, and emotional distress, medical expenses, and lost wages. The Paragard litigation is progressing, with bellwether trials scheduled for 2024 if settlements are not reached.
The bellwether trials are scheduled for 2024 if settlements are not reached. They will play a pivotal role in shaping the future of Paragard lawsuits. These trials will set important precedents and may establish the extent of legal liability and potential settlement amounts.
Final Thoughts
The Paragard lawsuit sheds light on the crucial importance of transparency, safety, and quality control in medical device manufacturing. The allegations against Paragard’s manufacturer highlight the need for comprehensive patient education regarding potential risks associated with contraceptive devices. Moreover, these cases underscore the profound impact that medical device complications can have on the lives of affected individuals.
The MDL in progress is a significant development, and the upcoming bellwether trials will play a major role. They may provide clarity on legal liability and establish precedents for settlements, impacting the broader conversation around medical device safety. These lawsuits serve as a reminder of the need for stringent regulatory oversight and quality assurance in the healthcare industry.